Abstract
Background Rabbit anti-thymocyte globulin (rATG) is used in allogeneic hematopoietic cell transplantation (HCT) to prevent graft failure through depletion of host T cells and decrease the risk of graft-versus-host disease (GVHD) through partial depletion of donor T cells. However, it has highly variable pharmacokinetics. Previous reports suggest rATG dosing should be based on recipient bodyweight and absolute lymphocyte count (ALC) prior to initiating rATG. This new dosing nomogram reduces rATG overexposure, potentially leading to faster immune reconstitution and subsequently superior overall outcomes.
Methods Since January 12, 2017, we use a previously described rATG dosing nomogram based on bodyweight and ALC before administering rATG in patients with inborn errors of metabolism receiving an umbilical cord blood transplant at the University of Minnesota Masonic Children's Hospital. The proposed rATG dosing varied from 2 to 10 mg/kg based on bodyweight and recipient ALC prior to initiating rATG, starting 8 or 9 days before HCT. The myeloablative conditioning regimen was Busulfan based, with addition of Fludarabine and Cyclophosphamide for lymphodepletion, as well as Rituximab and/ or Bortezomib for prevention of immune-mediated cytopenia. For GVHD prophylaxis cyclosporin A or tacrolimus were used with methylprednisolone. The primary endpoint was CD4+ immune reconstitution (>50 CD4+ T-cells/mL) within 100 days after transplantation. Secondary endpoints included overall survival, graft failure, acute and chronic GVHD, myeloid and T cell donor chimerism at one year post transplant, time to neutrophil engraftment and viral reactivations of cytomegalovirus, adenovirus and Epstein Barr virus. The study protocol was approved by the Institutional Review Board of University of Minnesota.
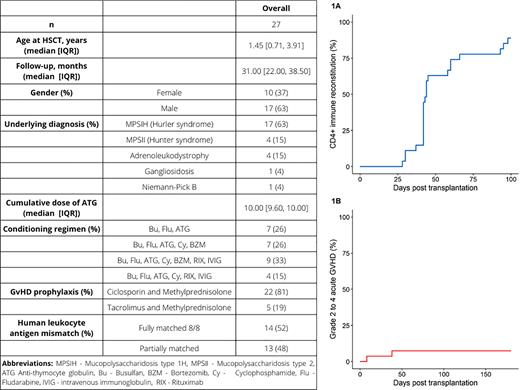
Results Between February 20, 2017, and June 28, 2021, 27 patients were included in the study. Median follow-up time was 31 months (IQR 22.0 to 38.5) and median age was 1.45 years (IQR 0.7 to 3.9). Ten (37%) of 27 patients were female. The baseline characteristics are presented in Table 1. rATG dose was adjusted below 10 mg/kg recipient body weight in 7 (26%) of 27 patients on average by 29% (SD 14.9%). The CD4+ recovery at 100 days post HCT was reached in 22 (85%) of 26 patients (Figure 1A). Overall, four (15%) patients died resulting in overall survival of 83% (95%CI 67% to 100%). Causes of death were intracranial bleeding due to immune-mediated thrombocytopenia (this patient was transplanted before introduction of bortezomib and rituximab for immune-mediated cytopenia prevention), diffuse alveolar hemorrhage, respiratory syncytial virus infection and disease progression. Median time to neutrophil engraftment was 14 days (IQR 12.5 to 18.0), and no graft failure was observed. All surviving patients had full myeloid chimerism and 14 of 23 (61%) of patients had full T cell donor chimerism at one year post HCT. Two (7%) patients developed acute GVHD grade II to IV (Figure 1B), and no patients had chronic GVHD. Six patients (22%) had cytomegalovirus viremia, five of them were seropositive before transplant, one patient was seronegative as was the mother of the donor. One patient, who was seronegative before transplant, had Epstein-Barr virus reactivation requiring treatment, no patient had adenovirus reactivation.
Conclusion In pediatric patients with inborn errors of metabolism individualized dosing of rATG was associated with a robust and early CD4+ immune reconstitution, with low therapy related mortality, low GVHD incidence compared to historical findings and stable full myeloid chimerism. These results are in line to the findings of the PARACHUTE trial previously published. However, the PARACHUTE trial included only small number of patients with inborn errors of metabolism.
Disclosures
Gupta:Blue Rock Therapeutics: Membership on an entity's Board of Directors or advisory committees; Vertex Pharmaceuticals: Consultancy. Boelens:Medexus: Consultancy, Honoraria; Omeros: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Advanced Clinical: Honoraria, Other: data safety monitoring board; Equillium: Consultancy, Honoraria; Sobi: Consultancy, Honoraria; SmartImmune: Consultancy, Honoraria; Race Oncology: Consultancy, Honoraria; Avrobio: Consultancy, Honoraria; BlueRock: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal